Toxins in Poison Dart Frogs
| As we have just seen, Poison Dart frogs contain a variety of toxins. Below I have listed and described a few of the more common ones. Most of the toxins within Poison Dart frogs affect the contraction of muscle cells or interfere with the transmission. For muscle contraction to occur, it must usually receive a electrical impulse from the surrounding muscle nerves. This impulse is carried down the axon of the neuron and then the signal is passed onto the next nueron across the snyaptic cleft (Campbell 1993). For that signal to be carried across nuerotransmitters bind with ion channels allowing ions such as sodium, potassium and chlorine to cross the membrae. This change of ions allows a change of membrane potential and ultimately the passing on of the signal to the next neuron (Campbell 1993). Most of the toxins in Poison Dart frogs affect with this transmission of signals towards muscle fibers.
|
||
|
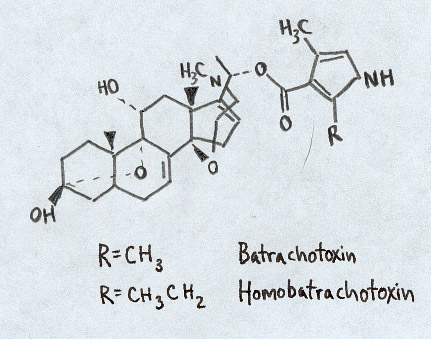
Batrachotoxins Most famously found in Phyllobates terriblis (left), this series of toxins is not to be ignored. Found throughout the genus Phyllobates, the concentration of toxin ranges wildly from virtually undetectable in some members all the way up to a whopping 1.9 mg in P. terriblis (Daly et al. 1980). The minimum lethal dose in a 20-gram mouse is .05 mg and at the average 1100 mg of compounds per frog, a single P. terriblis has enough poison to kill upwards of 20,000 mice (Patocka et al. 1999). These incredibly potent chemicals have the ability to "selectively increase the permeability of the outer membrane of nerve and muscle cells to sodium ions" (Myers and Daly 1983). By preventing the normal closing of these channels within muscle fibers it permits a large influx of sodium into the cell causing an irreversible electrical depolarization. Consequently, nerve signals which under normal circumstances would allow the muscle to relax are blocked and the muscle remains in a contracted state (Myers and Daly 1983). It turns out that the Purkinje cells within the heart are extremely sensitive to this fiber (Albuquerque et al. 1971). Consequently, the toxin causes heart arrhythmias, fibrilation and ultimately failure. In higher doses within mice, death has occured within a minute (Patocka et al. 1999). |
Chemical structure of Batrachotoxin. Figure adapted from Daly et al., 1997
Phyllobates terriblis. Photo courtesy of Dirk Huppert. |
|
|
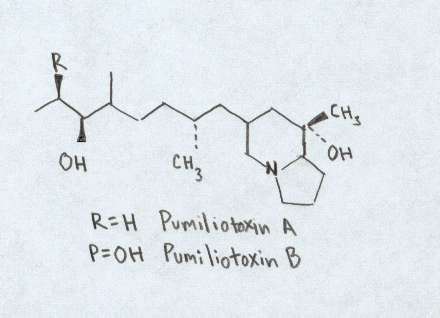
Pumiliotoxins Occuring in all species of Phyllobates and Dendrobates, over 100 toxins have been placed in this group. Despite the numerous toxins in this group, much is still unknown about this group of toxins. Divided into three groups, Pumiliotoxins Aand B are significantly more toxic than the C group. It appears that this group of toxins affect the transport of calcium ions in the calcium and sodium dependent processes withing nerve and skeletal muscles (Myers and Daly 1983; Patocka et al. 1999). For comparisons sake, the pumiliotoxins are often 100 to 1000 times less toxin than their batrachotoxin counterparts (Patocka et al. 1999).
|
Chemical structure of Pumiliotoxin. Figure adapted from Daly et al., 1997
Dendrobates pumilio. Photo courtesy of Dirk Huppert. |
|
|
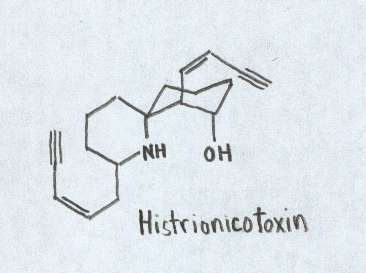
Histionicotoxins This unusual set of toxins has the ability to create a blockage of ions between the end-plate channel and the acetylcholine receptor (Myers and Daly 1983). The toxin prevents ions from flowing out of nerve and muscle cells thereby preventing them from returning to the resting state. This in turn lengthens the transmission of nerve muscles and consequently prolongs muscle contraction (Myers and Daly 1983). It is not certain as to where these toxins are derived as of yet. It is believed they may be found in some Neotropical ants (Daly et al. 1997).
|
Chemical structure of Histrionicotoxin. Figure adapted from Daly et al., 1997
Dendrobates histrionicus. Photo courtesy of Dirk Huppert.
|
|
|
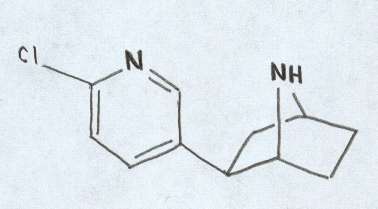
Epibatidine This rare alkaloid was first found in Epipedobates tricolor. It has been shown to be 200 times more potent than morphine. However, the pain relieving effects were not blocked by naloxone like they are in morphine (Daly et al. 1997). The source of this alkaloid is still unknown but it is believed to be tied to a small arthropod within the leaf litter (Daly et al. 1997). |
Chemical structure of Epibatidine. Figure adapted from Daly et al., 1997.
Epipedobates tricolor. Photo provided by Dirk Huppert. |
|